AG Schmidt
Understanding uptake and action of bacterial protein toxins
We study the molecular mechanisms of several bacterial protein toxins, which are often very efficient enzymes. They covalently modify Rho GTPases by different modifications which lead to permanent activation of Rho proteins or to inhibition of downstream signaling. Therefore, we use bacterial toxins as tools to study Rho-dependent physiological and pathophysiological signaling pathways.
Key Papers:
1. Reipschläger, S., Kubatzky, K., Taromi, S., Burger, M., Orth, J., Aktories, K., Schmidt, G. (2012). Toxin-induced RhoA activity mediates CCL1-triggered STAT signaling. J. Biol. Chem, 287(14), 11183-11194.
2. Augspach, A., List, J. H., Wolf, P., Bielek, H., Schwan, C., Elsässer-Beile, U., Aktories, K., Schmidt, G. (2013). Activation of RhoA,B,C by Yersinia Cytotoxic Necrotizing Factor (CNFy) Induces Apoptosis in LNCaP Prostate Cancer Cells. Toxins, 5(11), 2241-2257.
3. Lang, S., Busch, H., Boerries, M., Brummer, T., Timme, S., Lassmann, S., Aktories, K., Schmidt, G. (2017). Specific role of RhoC in tumor invasion and metastasis. Oncotarget, 8(50), 87364-87378.
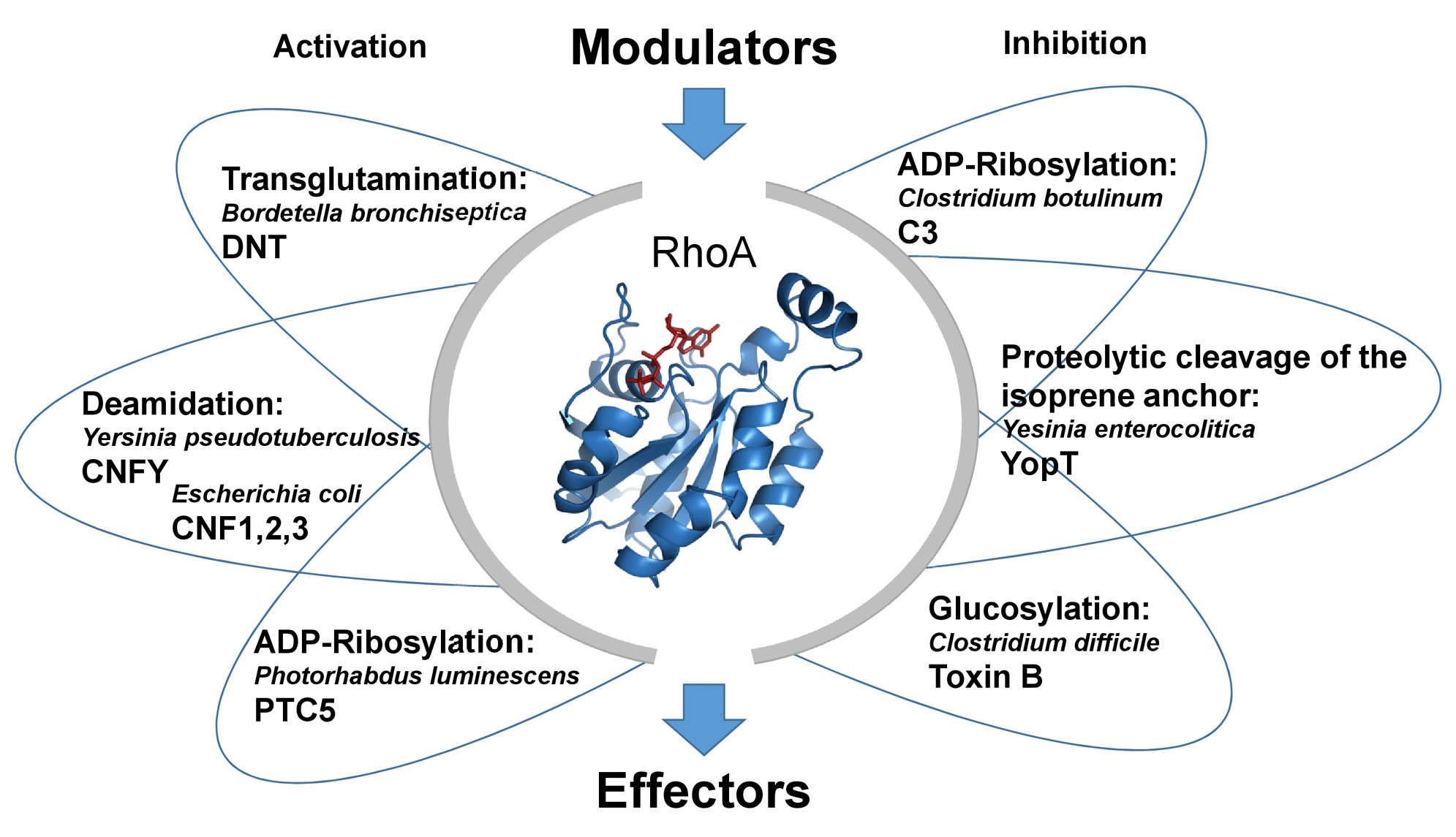
Several bacterial protein toxins catalyze covalent modifications of Rho proteins, thereby leading to permanent activation or inhibition of Rho signaling.
Bacterial toxins are taken up by receptor-mediated endocytosis. In recent years, several cellular membrane proteins have been identified which bind with high affinity and specificity to the toxins and mediate their endocytosis into mammalian cells. We are interested in the question, whether bacterial toxins may activate or block signaling pathways downstream of their receptors. Moreover, we intend to engineer toxins with changed and defined cellular specificity with the aim to use the efficient and specific enzymes as potential pharmacological agents, for example to block movement of cancer cells.
Key Papers:
1. Piteau, M., Papatheodorou, P., Schwan, C., Schlosser, A., Aktories, K., Schmidt, G. (2014). Lu/BCAM Adhesion Glycoprotein Is a Receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1). PLoS Pathog, 10(1), e1003884.
2. Schmidt, G., Papatheodorou, P., Aktories, K. (2015). Novel receptors for bacterial protein toxins. Current Opinion in Microbiology, 23, 55–61.
3. Zahaf, N. I., Lang, A. E., Kaiser, L., Fichter, C. D., Lassmann, S., McCluskey, A., Augspach, A., Aktories, K., Schmidt, G., (2017). Targeted delivery of an ADP-ribosylating bacterial toxin into cancer cells. Sci Rep., 7, 41252.
4. Reppin, F., Cochet, S., El Nemer, W., Fritz, G., Schmidt, G. (2018). High affinity binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM adhesion glycoprotein. Toxins, 10(1), 3.

Many bacterial protein toxins are taken up into mammalian cells by receptor-mediated endocytosis (modified from Schmidt et al., Current Opinion in Microbiology, 2015.)
The entomopathogenic bacterium Photorhabdus luminescens produces an autonomous protein injection machinery. One of our aims is to engineer this machinery for injection of diverse proteins and molecules into mammalian cells.
Key Papers:
1. Lang, A. E., Schmidt, G., Schlosser, A., Hey, T. D., Larrinua, I. M., Sheets, J. J., Mannherz, H. G., Aktories, K. (2010). Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science, 327(5969), 1139-1142.
2. Aktories, K., Schmidt, G., Lang, A. E. (2015). Photorhabdus luminescens Toxins TccC3 and TccC5: Insecticidal ADP-Ribosyltransferases that Modify Threonine and Glutamine. Curr Top Microbiol, 384, 53-67.

The trimeric toxin complex (TC) from Photorhabdus luminescens is composed of a catalytic part (TcC), an adaptor protein (TcB) which mediates contact to TcA and loading of TcC into TcA. TcA is the injection machinery and mediates cell binding. The structure of the protein complex was solved recently by crystallography and cryo-electronmicroscopy. (Lang, Schmidt et al., Science, 2010)

